Fixation Protocol
Perfusion fixation
If it is possible, perfusion is the preferred method of tissue preservation. These are the materials that you will need for perfusion fixation:
| Name | Vendor | Catalogue Number |
| 32% Paraformaldehyde | Electron Microscopy Sciences | 15714-S |
| 50% Glutaraldehyde | Electron Microscopy Sciences | 16310 |
| 10X PBS | Thermo Fisher Scientific | 70013-073 |
Create a solution with a final concentration of 1X PBS, 4% paraformaldehyde (PFA), and 1% glutaraldehyde (GA). As 40 mL of this solution is necessary for each perfusion, a typical recipe is: 4mL 10X PBS, 5 mL 32% PFA, 0.8 mL 50% GA, and 30.2 mL water. This solution should be made fresh immediately prior to performing perfusion and kept on ice at all times. It is recommended to chill all of the separate ingredients before mixing the components.
Using the perfusion technique of your choice, first perfuse 20 mL of ice-cold PBS through the beating heart of an anesthetized mouse, followed by 20 mL of the ice-cold perfusion solution described above. Take care not to introduce any bubbles during the procedure, and use a flow rate slow enough to avoid damage to the vasculature or brain sample (<5 mL/min). After both solutions have been perfused, carefully remove the brain from the skull using any technique you are comfortable with. The dura membrane should also be removed during the process. Place the sample into 20 mL of perfusion solution and incubate at 4 ˚C with gentle shaking for 3 days.
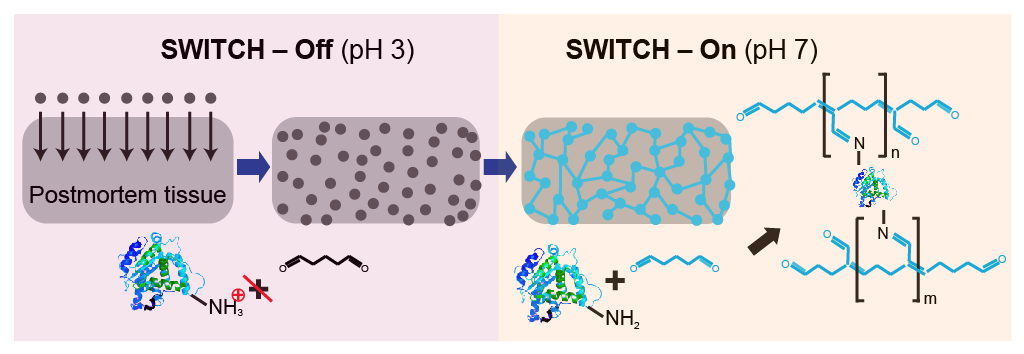
SWITCH fixation
You should use SWITCH fixation if you can't do perfusion with the 4% PFA, 1% GA solution.
SWITCH fixation involves two steps: SWITCH-Off at pH 3 to disperse the fixatives (fixatives don't work well at pH 3) and SWITCH-On at pH 7 to begin fixing the tissue.
These are the materials that you will need for SWITCH fixation:
| Name | Vendor | Catalogue Number |
| 32% Paraformaldehyde | Electron Microscopy Sciences | 15714-S |
| 50% Glutaraldehyde | Electron Microscopy Sciences | 16310 |
| 10X PBS | Thermo Fisher Scientific | 70013-073 |
| 37% Hydrochloric acid | Sigma Aldrich | 320331-500ML |
| Potassium hydrogen phthalate | Sigma-Aldrich | P1088 |
If you are using PFA-fixed tissues (e.g. tissues from tissue banks), you need these two solutions:
- SWITCH-Off Fixative: 1X PBS, 4% GA, 0.05 M KHP, titrated with HCl to pH 3.
- SWITCH-On Fixative: 1X PBS, 1% GA.
Tips for making the SWITCH-Off Fixative: Titrate a bottle of PBS to pH 3 using HCl. Create solutions of 0.1 M HCl in water and 0.1 M potassium hydrogen phthalate (KHP) in water. Finally, mix these solutions in a ratio of 2:1:1 (pH 3 PBS):(0.1 M HCl):(0.1 M KHP). To this new solution, add a stock solution of GA to make a final concentration of 4% GA. Ensure that this solution stays cold at all times. It is recommended to chill the solution before adding GA.
You need to add the KHP to the SWITCH-Off Fixative to undo some of the PFA fixation.
(If you are using fresh tissues (not fixed previously), we recommend you first fix with PFA for several days before proceeding. This is because tissues can degrade quickly and because PFA fixation is quick and does not require SWITCH processing.)
Once you're ready, incubate the sample in 40 mL fixation-OFF solution at 4 ˚C with gentle shaking for 2 days. The sample should then be moved to fixation-ON solution at 4 ˚C with gentle shaking for an additional 2 days. (The timing for the Fixation OFF and ON steps is dependent on the sample size and may need to be optimized from these starting values on a case-by-case basis. We found that these parameters worked well for banked human samples of roughly 0.5-1.0 cm thickness.)